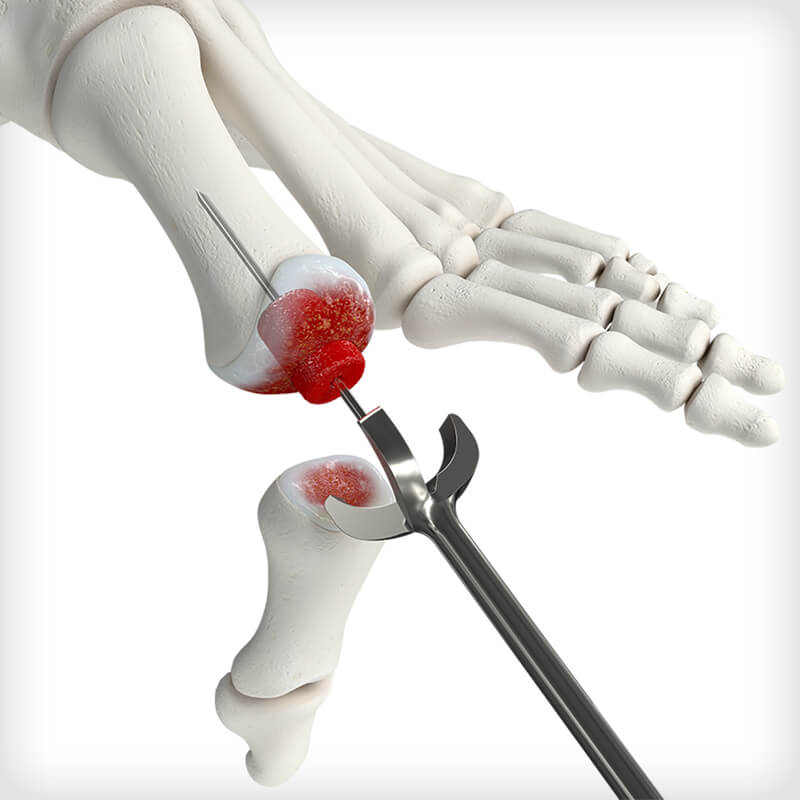
Forefoot
Pre-hydrated MTP Revision Bioimplants
Manufacturer: Medline
Healthcare Systems: Arizona Care Network,CommonSpirit Health,HonorHealth,Sovereign Healthcare,Steward Health Care,USPI,VA Heatlhcare System,Atlas Healthcare Partners,SurgCenter Development,Banner Health
Procedure: 1st MTP Fusion
Dense cancellous MTP Revision Bioimplants are pre-hydrated in saline to eliminate idle OR time associated with cutting and soaking a traditional bone graft. The bioimplants are sterilized in saline rather than freeze-dried, to preserved the structural integrity of the graft and to avoid intraoperative graft fracture when reaming. Two distinct size options are available for unique revision scenarios: a small option designed to backfill metatarsal head voids and a large option design to bridge a larger gap and restore length. The bioimplants feature a flat-cut, cannulated designed to be used with a provided 1.6mm guidewire, MTP cup and cone reamers, and acorn reamer. Reamers are used for intraoperative shaping and graft site preparation, which may vary depending on revision scenario and surgeon-preference or technique.